ABLi Therapeutics Reports Final Results from the Phase 2 ‘201 Trial’ Evaluating Risvodetinib for the Treatment of Parkinson’s Disease
Risvodetinib demonstrated to be safe and well-tolerated with preliminary clinical benefits
Risvodetinib demonstrated to be the first treatment to reduce alpha-synuclein pathology, widely recognized as the underlying cause of Parkinson’s disease
Blood-borne biomarkers evaluate target engagement and mechanism of action to confirm the Company’s underlying rationale for treatment
ATLANTA and BOSTON, Oct. 09, 2025 (GLOBE NEWSWIRE) -- ABLi Therapeutics (“ABLi”), a biotechnology company developing therapeutics to address diseases that arise from activation of Abelson Tyrosine Kinases (c-Abl kinases), announces final trial results from the Phase 2 “201 Trial” (NCT05424276) evaluating risvodetinib in patients with untreated Parkinson’s disease (PD), meeting its primary endpoint in safety and tolerability demonstrating risvodetinib safe and well-tolerated with a side-effect profile similar to placebo. These results were presented as the “Clinical Breakthrough Lecture” during the keynote session at the 2025 Movement Disorders Society Annual Congress.
The 201 Trial is the first long-term dosing trial evaluating the selective, brain-penetrant c-Abl kinase inhibitor risvodetinib as a monotherapy in untreated PD. The trial evaluated a 50 mg, 100 mg or 200 mg dose in 126 participants randomized 1:1:1:1 to either a dose of risvodetinib or placebo given once daily for 12 weeks. Risvodetinib’s human exposure is two-fold to 200-fold higher than any c-Abl inhibitor currently approved for human use, necessitating the need to establish safety at 12-weeks before embarking on a registrational studies to measure the effect of Risvodetinib on the progress of disease.
Risvodetinib met the primary endpoint in safety and tolerability:
- The fraction participants that experienced one or more adverse events and the average number of adverse events per person were similar to placebo
- 95% of enrolled participants completed 12-week dosing with 99% dosing compliance
- Common adverse events such as nausea, diarrhea, vomiting, edema or cardiovascular events were no more frequent in participants administered any dose of risvodetinib as compared to participants administered placebo.
Risvodetinib impacted key secondary endpoints related to the Activities of Daily Living, the most important metric for evaluating clinical benefit:
- The MDS-UPDRS Part 1, Part 2 and the Schwab & England Activities of Daily Living (SEADL) scales favored treatment at all doses
- Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part 2 and the Schwab & England Activities of Daily Living Scale (SEADL) scales reached nominal statistical significance at the 100 mg and 50 mg doses, respectively.
- Frequency of falling reported as an adverse event reduced nearly 5-fold for risvodetinib treatment relative to placebo treatment
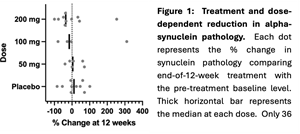
Exploratory endpoints demonstrated Risvodetinib reduced alpha-synuclein pathology in skin biopsy at all doses:
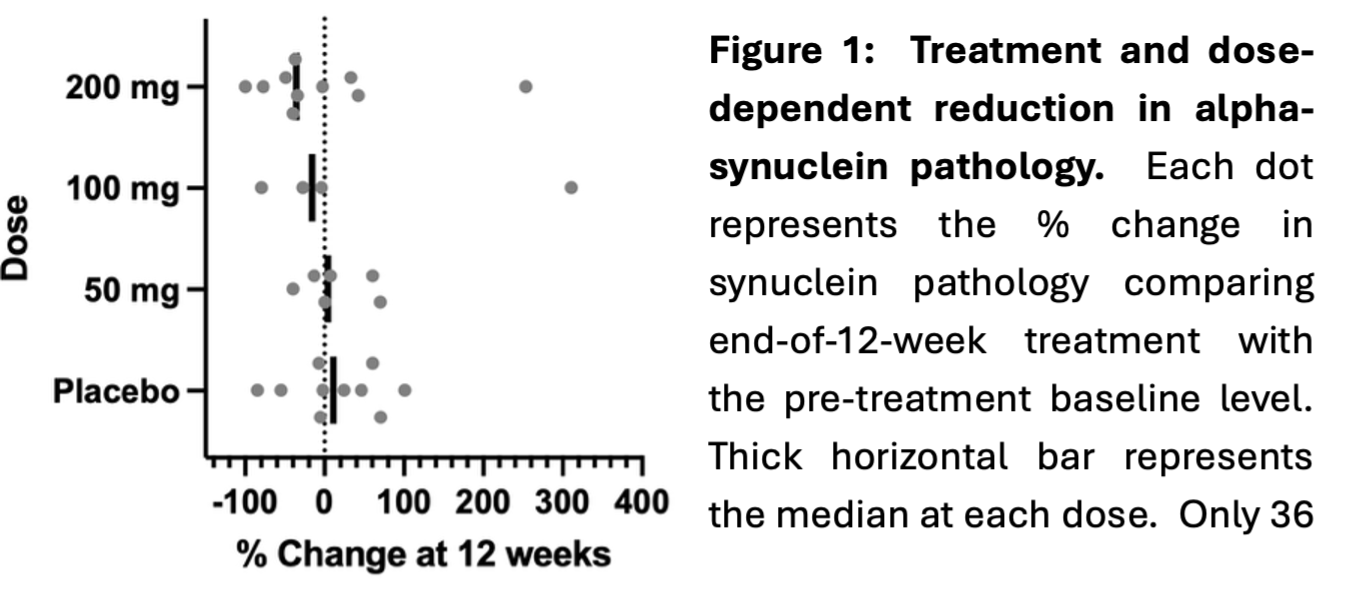
“The outcomes of the 201 Trial were wholly unexpected,” noted Dr. Milton Werner, Chairman & Chief Executive Officer of ABLi Therapeutics. “We had seen a good safety profile with 7-day dosing, but didn’t anticipate the drug would have an adverse event profile similar to placebo at longer dosing durations, with none of the typical side-effects of other drugs in the class. A preliminary insight into clinical benefit may have been seen from secondary functional assessments, but the trial was not designed to measure efficacy. We think the most important measure beyond safety is the confirmation that Risvodetinib can reduce the alpha-synuclein pathology that drives Parkinson’s disease. This outcome is the first measure of an experimental treatment reducing the disease-causing pathology of PD,” said Dr. Werner “Since the Trial’s completion, we have used new blood-borne biomarkers to establish that the underlying treatment rationale, first elucidated by ABLi and its collaborators from animal models, is also the active mechanism of neurodegeneration in humans.”
About Risvodetinib (ABLi-148009)
Risvodetinib is a potent, selective small-molecule inhibitor of the non-receptor c-Abl kinases, designed for once-daily oral use that targets the underlying biological mechanisms driving Parkinson’s disease initiation and progression. Risvodetinib is believed to be a disease-modifying therapy that halts disease progression and reverses the functional loss arising from Parkinson’s disease inside and outside of the brain. All marketed therapeutic approaches to treat Parkinson’s help manage the symptoms of the disease, but there are currently no available treatments to slow or stop the disease’s relentless progression. Recently, risvodetinib was the first monotherapy shown to improve patient quality of life in a randomized, placebo-controlled clinical trial (NCT NCT05424276) and simultaneously reduced the underlying synuclein aggregate pathology in untreated Parkinson’s disease. Risvodetinib currently has intellectual property protection beyond 2036.
About ABLi Therapeutics
ABLi Therapeutics (“ABLi”) applies innovative medicinal chemistry and a deep understanding of disease biology to develop small molecule therapeutics that target the cause of diseases that arise from activation or dysfunction of the Abelson Tyrosine Kinases (c-Abl). Leveraging its expertise in drug design, ABLi utilizes clinically validated data of kinase inhibitors to design and develop novel product candidates with enhanced penetration into the brain, greater potency and target selectivity, and improved safety to treat diseases in which Abl kinase activation or dysfunction is implicated. The Company’s primary focus is on developing therapeutics for the treatment of neurodegenerative diseases like Parkinson’s disease and the Parkinson’s-related neurodegenerative diseases Multiple System Atrophy and Dementia with Lewy Body that are all associated with Abl kinase activation or dysfunction. For more information visit www.ablitherapeutics.com or follow us on LinkedIn, Facebook or Instagram.
Contacts:
For ABLi Therapeutics
info@ablitherapeutics.com
Investor/Media
Mike Moyer
Managing Director – LifeSci Advisors
mmoyer@lifesciadvisors.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/637cb6e1-f930-4f10-8c69-62b5ec54645e

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.